Indonesia Joins Phase 3 TB Vaccine Trial Backed by Bill Gates
Indonesia joins the phase 3 trial of the new TB vaccine supported by Bill Gates. A major breakthrough towards the 2030 TB elimination goal.
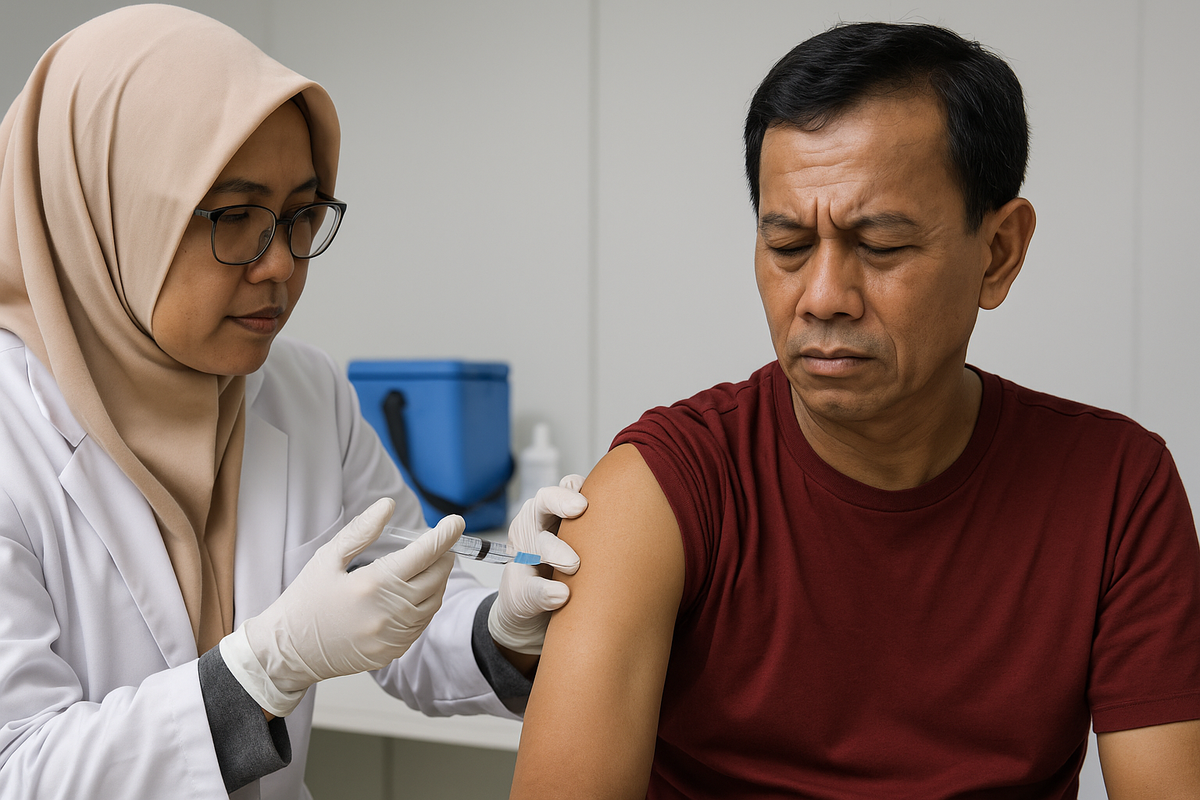
Indonesia Takes Part in Phase 3 TB Vaccine Trial
Indonesia is officially one of five countries participating in the phase 3 clinical trial of the M72/AS01E tuberculosis (TB) vaccine. The vaccine is developed by GlaxoSmithKline and backed by the Bill & Melinda Gates Medical Research Institute, as part of a global effort to combat TB—still one of the world’s deadliest diseases.
Phase 3 Clinical Trial: The Final Step Before Approval
Phase 3 represents the final stage of vaccine testing before regulatory approval. In Indonesia, the trial began in September 2024 and involves 2,095 adult participants with latent TB infections. Other participating countries include South Africa, Kenya, Malawi, and Zambia.
Government Support and Safety Assurance
Health Minister Budi Gunadi Sadikin emphasized that the entire process is closely monitored by BPOM, WHO, and international vaccine experts. So far, no serious side effects have been reported. The public is urged to understand that participation is voluntary and subject to strict safety protocols.
Strategic Benefits for Indonesia
Indonesia’s participation offers strategic advantages such as early access to the vaccine, potential for local production by Bio Farma, and knowledge transfer through partnerships with national universities like Unpad and UI. The M72/AS01E vaccine is projected to prevent up to 76 million TB cases and save USD 41.5 billion globally over the next 25 years.
Hope for 2030 TB Elimination
If proven effective, this will be the first successful TB vaccine since BCG was introduced over a century ago. The Indonesian government has set a target to eliminate TB by 2030, and this trial is a significant step toward achieving that goal.
Indonesia’s involvement in the M72/AS01E TB vaccine trial marks a bold step toward global TB control. From early access and domestic vaccine production to national elimination efforts, this initiative holds enormous promise if the vaccine proves successful.


Comments ()